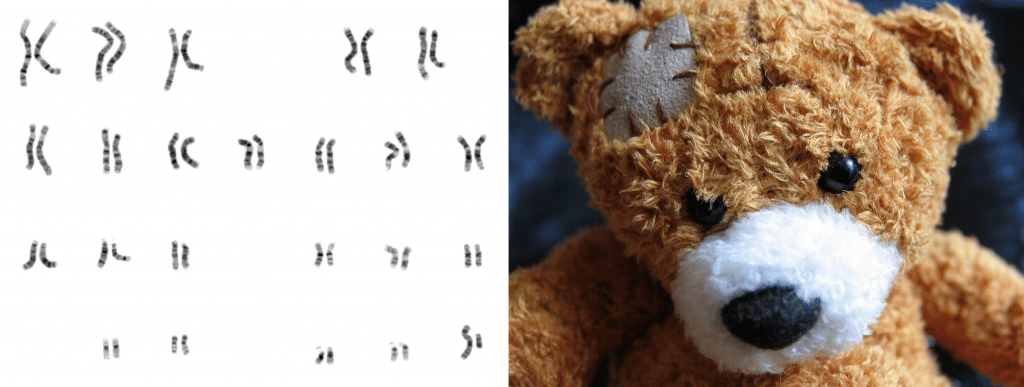Part of the Hallmarks of Aging series.
Did you know you have two different ages? The obvious one is chronological age, or the number of years since you were born. The other is biological age, or the time-dependent decline of your body’s function and appearance.
Chronological and biological age correlate with each other, so the signs of aging appear around a similar chronological age in most people. But often people exhibit signs of biological aging at very different rates. Some 70-year-olds run marathons, write great books, and are as active and productive as their 40 year old selves were, while many others of the same age can’t care for themselves and suffer from serious diseases like diabetes, cancer and dementia. An even more extreme example can be found in progeroid conditions–congenital disorders that cause the signs of biological aging to begin at a very young age.
In recent years, scientists studying the molecular and cellular processes that govern these changes and their variation in individuals have identified nine interconnected “hallmarks of aging”. Determined mainly by our genetics, but modulated by environmental factors, each of these nine hallmarks contributes to the damage that occurs with age and ultimately drives age-associated pathologies.
I. Genomic instability
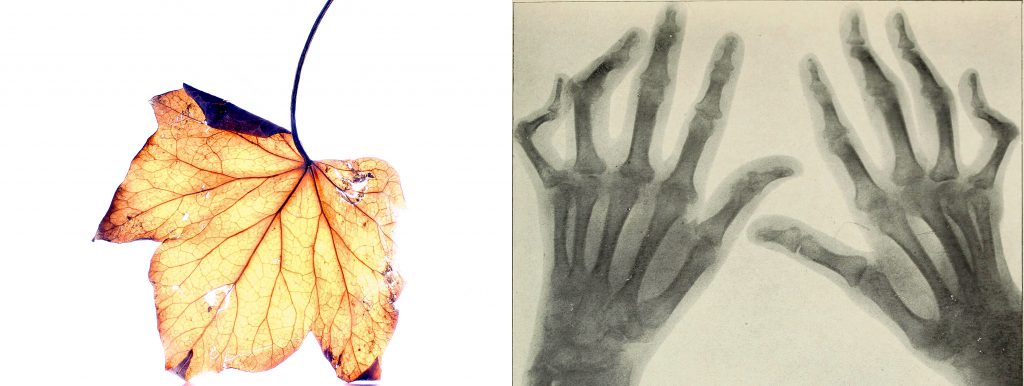
Exposure to smoke, chemicals or other exogenous agents over time can damage our genome, as can factors like simple DNA replication errors or oxidative stress. Although we have evolved a complex network of DNA repair mechanisms, DNA damage accumulates over the course of our lives, causing mutations in cells that can in the worst cases lead to cancer formation.
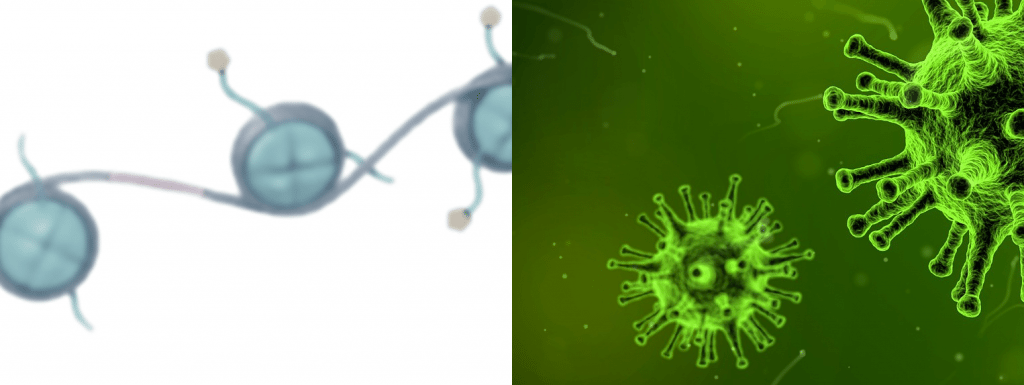
II. Telomere attrition
Similar to the plastic tips of shoelaces protecting their braided ends, telomeres protect the terminal ends of our chromosomes from deterioration. The normal DNA replication mechanisms in most of our cells are not able to copy the ends of our DNA completely, so the repetitive DNA sequences of the telomere region shorten with each cell division. After a number of replications, this leads to cell growth arrest, limiting the ability of tissues to regenerate as we age.
III. Epigenetic alterations
You may wonder how our various tissues and organs can appear so different from one another, since the genetic information encoded in our DNA is exactly the same in all cells in our body. In fact, DNA is modified with epigenetic information that enhances or suppresses the expression of particular genes as required by different tissue types. For example, if a cell should develop into a liver cell, epigenetic modifications will ensure that the parts of the genome specific to liver cells are expressed, while the parts specific to other cell types are ignored. The aging process often involves changes in our epigenetic code, which can lead to changes in gene expression that affect normal cellular function. In the immune system, for example, this could shift the balance between activating and suppressing immune cells, causing our bodies to be less resilient to pathogens.
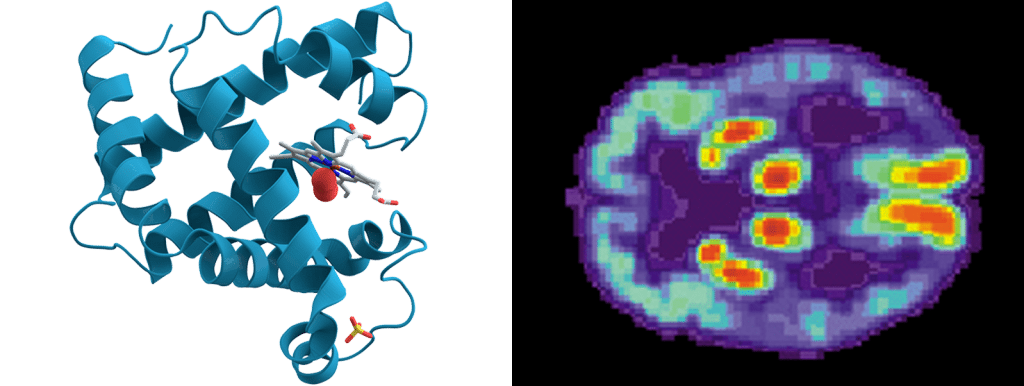
IV. Loss of proteostasis
In our cells, proteins are constantly being synthesized and degraded in a process known as protein homeostasis, or proteostasis. Proteins are like tools which must be assembled in the correct way to perform their many crucial cellular functions, and an important part of assembly is folding proteins into the proper shapes. Various mechanisms have evolved to better stabilize or restore correctly folded proteins, and to remove and degrade improperly shaped proteins which could otherwise accumulate and damage the cell. When these mechanisms become less efficient over time, damaged or aggregated protein components cause dysfunction or even cell toxicity, as seen in diseases like Alzheimer’s.
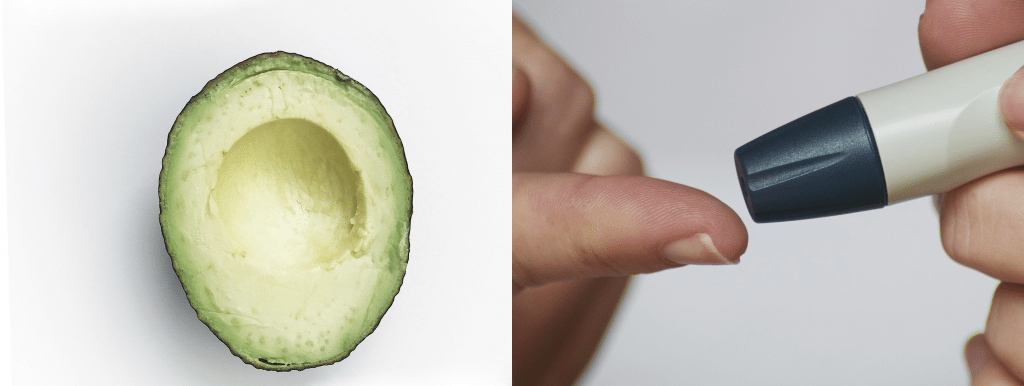
V. Deregulated nutrient sensing
When nutrients are abundant, cells and tissues respond by storing energy and growing, while nutrient scarcity activates homeostasis and repair mechanisms. In diabetes and obesity, cells are exposed constantly to abundant nutrients, causing the cellular mechanisms that sense nutrients to become desensitized. Deregulation of the nutrient sensing pathway also occurs during aging, and as a result, cells fail to respond properly to the cues that normally regulate energy production, cell growth, and other crucial cell functions.
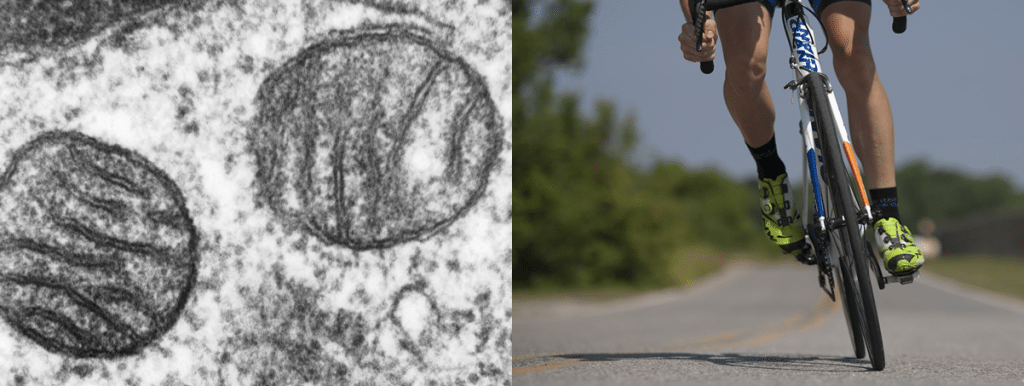
VI. Mitochondrial dysfunction
Free radicals, or reactive oxygen species (ROS), form a natural byproduct of energy production in the mitochondria. Although they have a role in cellular signaling, in high doses free radicals can be damaging to the cell. The free radical theory of aging proposes that over time, increasing ROS production triggers mitochondrial dysfunction, which causes further increases in ROS production and cellular deterioration. Mitochondrial dysfunction may also affect other important cellular signalling pathways and processes. Eventually the cell becomes less efficient at producing energy, and at the same time, levels of oxidative stress increase, causing damage to other cellular components. As a result, mitochondrial dysfunction contributes to various age-related conditions such as myopathies and neuropathies.
VII. Cellular senescence
Once cells are subjected to enough stress, DNA damage and telomere shortening, they enter a state of stable growth arrest called cellular senescence. This is a protective measure to prevent cells with genomic damage from becoming cancerous, but it also prevents old, worn out tissues from being replenished. Senescent cells change dramatically in their function, most importantly in the molecules they secrete, often pro-inflammatory molecules that damage the environment of the cells. This leads to chronic tissue inflammation that probably contributes to a variety of geriatric conditions like osteoarthritis and kidney dysfunction.
VIII. Stem cell exhaustion
One of the most obvious consequences of growing old is worse recovery from injury, caused by a decline in the ability of our stem cells to replenish damaged tissues. Your stem cells spend most of their time dormant in a niche, but as they are activated to heal wounds and restore tissues, they are also susceptible to telomere shortening, DNA damage and cellular senescence. Over time this results in stem cell exhaustion.
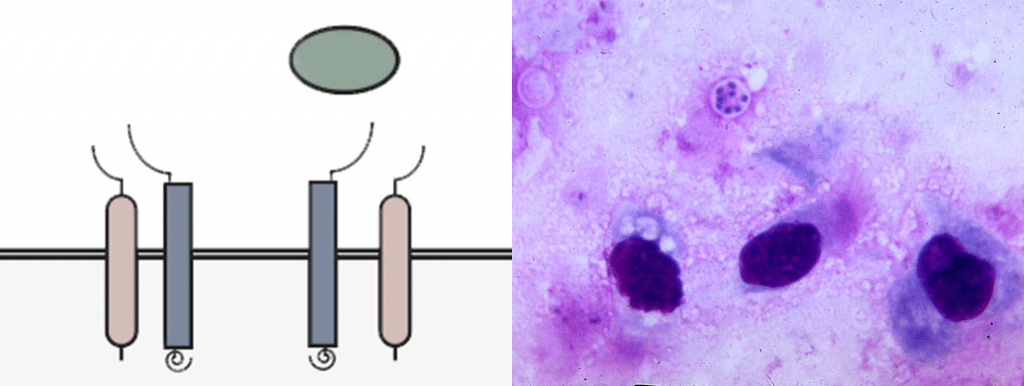
IX. Altered intercellular communication
In order to grow and function normally, our cells must constantly transfer information to each other, secreting signaling molecules to their neighboring cells or even sending molecular messengers through the bloodstream to affect cells and tissues far away. Aging changes not only the signals that are sent by cells, but also the ability of receiving cells to respond to such signals. This dysfunctional communication leads to issues like chronic tissue inflammation, as well as failure of the immune system to recognize and clear pathogens or dysfunctional cells, increasing susceptibility to infection and cancer.
Can we influence these hallmarks to prevent age-related damage and disease?
While our understanding of these complex processes is still limited, scientists and biotech entrepreneurs are hopeful that they’ll shed light on new strategies for combating aging. Already some are optimistic about potential drug therapies such as CR mimetics, which may improve nutrient sensing, and senolytics, a class of drug that removes senescent cells.
Next we’ll go in depth on genomic instability, its role in aging damage and what scientists think we can do about it.